Decarbonisation & Carbon Capture utilisation & Storage (CCUS)
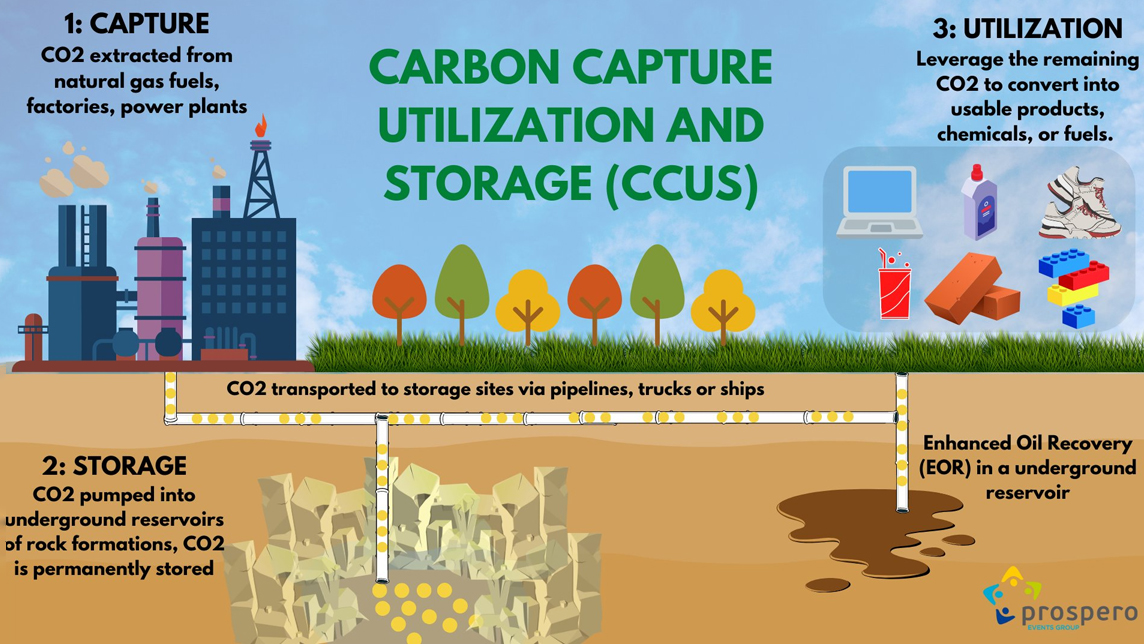
Globally, power and industry account for about 50 per cent of all greenhouse gas (GHG) emissions. Carbon Capture, Utilization, and Storage (CCUS) encompasses technologies to stop CO2 from being released to the atmosphere, followed by upcycling the CO2 for utilization and determining safe and permanent storage options, thereby reversing its negative impacts.
To enable circular carbon economy (CCE), the Carbon Capture technology helps plant operators to capture Carbon dioxide (CO2) at the point of emission, i.e. process emissions and chimneys using chemical and physical separation processes. The captured CO2 can be further used for:
- 1. Enhanced Oil Recovery (EOR) for extraction of crude oil from an oil field that cannot be extracted otherwise.
- 2. Production of fuels like Methanol and derivatives, plastic components, fire extinguishers, pharma and soda ash.
- 3. Food and beverages industry.
- 4. Building material aggregates and agriculture.
Further innovations in this area include artificial photosynthesis using bio-solar leaves and phytoplankton-based solutions that mimic the chemical process of photosynthesis. Also, the captured CO2 can be stacked and stored deep inside geological formations such as exploited oil and gas wells.
CCUS technologies can play an important role in meeting Net Zero targets and is one of the key pathways to reduce emissions while continuing sustainable development at an unprecedented pace.
Carbon Capture, Storage (CCS) and Utilisation (CCUS) are important emission reduction technologies and can help to scale up the hydrogen economy (Blue & Green Hydrogen). Depleted gas reservoirs represent a great opportunity for underground CO2 storage. They provide significant storage capacity as well as infrastructure in place. Benefit from our experience in developing underground storage facilities. We have adopted a robust workflow starting from identifying CCS opportunities to maturing these to underground storage projects.
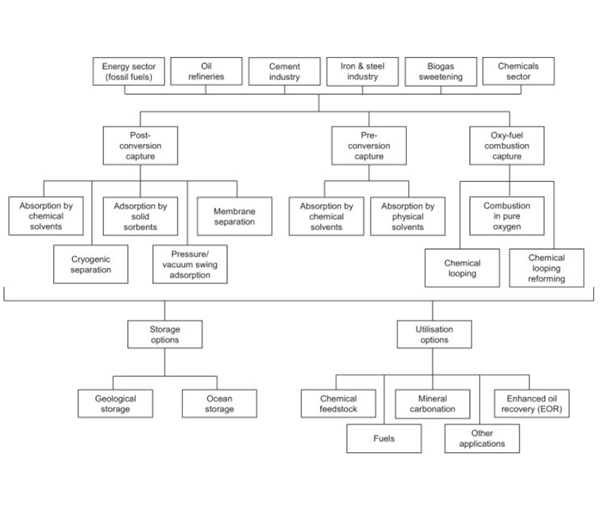
Carbon capture technologies remove carbon dioxide from flue gases emitted from industrial production processes. The two major CO2 capture technologies, viz. pre-combustion capture and post-combustion capture systems. Captured CO2 can be used for use in enhanced oil recovery, gas recovery, soda ash manufacturing and the food and beverage industry.
Let’s have look at some of the commercially proven Carbon Capture technology processes:
- 1. Pre-combustion capture: When CO2 is captured from a pressurized, reducing environment process gas stream. In this process, the CO2 is usually captured by chemical/physical absorption in a solvent and subsequently released by thermal regeneration which can be further compressed for transport and storage. Depending on the end-user requirement, syngas composition is changed through reformer and water-gas shift reactors. The industrial processes that already require the generation of hydrogen or synthesis gas, pre-combustion capture is the preferred route.
- 2. Post-combustion capture: When CO2 is captured in a low pressure, low CO2 concentration, and oxidizing environment flue gas stream. This process separates CO2 from the exhaust gases after combustion (like during iron and steel making processes, Power Plants, SMR). CO2 can be captured using a liquid solvent like monoethanolamine (MEA). After absorption in the solvent, the CO2 is released by thermal regeneration to obtain a high-purity stream of CO2.
The major difference between pre-combustion and post-combustion is that the former is favored in cases where the gas stream has higher partial pressure of CO2, such as in gasification or sour gas processing. Since no chemical bonds need to be broken for solvent regeneration, the thermal energy penalty is much lower. The regeneration of the physical solvent is achieved mainly by reducing pressure.
2. Post-combustion capture: When CO2 is captured in a low pressure, low CO2 concentration, and oxidizing environment flue gas stream. This process separates CO2 from the exhaust gases after combustion (like during iron and steel making processes, Power Plants, SMR). CO2 can be captured using a liquid solvent like monoethanolamine (MEA). After absorption in the solvent, the CO2 is released by thermal regeneration to obtain a high-purity stream of CO2.
The major difference between pre-combustion and post-combustion is that the former is favored in cases where the gas stream has higher partial pressure of CO2, such as in gasification or sour gas processing. Since no chemical bonds need to be broken for solvent regeneration, the thermal energy penalty is much lower. The regeneration of the physical solvent is achieved mainly by reducing pressure.
- 3. Oxyfuel Combustion: It is a process of burning the fuel with nearly pure oxygen instead of air. Combustion air in a power plant is replaced with pure oxygen. This produces pure stream of CO2 as flue gas stream. To control the flame temperature, some part of the flue gas is recycled back into the furnace/boiler.
The principles of Oxyfuel Combustion can also be used in the steelmaking and the cement-making industries. Oxyfuel Combustion has been demonstrated at pilot scale on coal-fired boilers in Germany, Spain and Australia.
- 4. Direct Air Capture: As name suggests, Direct Air Capture extracts CO2 directly from atmosphere and stores it permanently away from the atmosphere. However, it is energy intensive as of now as locating one CO2 molecule out of 2500 molecules of air requires lot of energy. Recently commissioned Orca’ direct air capture and storage facility, Iceland will remove 4,000 tons of carbon dioxide annually.
Time to act is now. Else if you are young enough to be around in 2025, you will witness more frequent extreme events such as more powerful hurricanes, rise in food prices, floods leading to the movement of millions of climate refugees.